By the end of glycolysis and the Krebs Cycle, the glucose molecules are fully consumed. Carbon dioxide, 4 ATPs, 2 FADH, and 8 NADH have been produced. The reduced coenzymes (FADH and NADH) carry some electrons from glucose, and they are full of potential energy that need to extracted in order to recycle these molecules, and also to produce way more ATP. In this post we will see an general idea of what happens to these coenzymes and how different NADH is from FADH.
Coenzymes do not directly react with oxygen. The electrons carried by NADH are passed though different electron carriers until it reach the terminal electron receptor, oxygen. In fact, oxygen also picks up some water from the surrounding and forms water. Most of the electron carriers are embedded inside of the membrane (bacterias - cell membrane, and eukaryotes - mitochondrial membrane.)
The free energy resulted from this redox reactions is used in the phosphorylation of ADP. The overall process is known as "Oxidative Phosphorylation" or simply OxPhos.
Storing Energy in the Electrochemical Gradient
The potential energy acquired during the redox reactions of OxPhos must be stored temporarily before it is used to make ATP. This is accomplished by pumping hydrogen ions across the membrane (prokaryotes - plasma membrane, eukaryotes - mitochondrial membrane.) Enzymes that are responsible to catalyse the transfer of electron from one electron carrier to another (redox reactions) also helps to pump hydrogen ions across the membrane. Pumping of hydrogen requires active energy, because it is moving against its gradient in order to store potential energy in the electrochemical gradient.
Energy for pumping hydrogen ions
As we have previously seen, the process of transferring hydrogen atoms across membrane is a active process, but where does the energy comes from? It comes from secondary active transport. Thus, it acquires energy from other redox reactions that occur between electron carriers.
NADH transfer its electron to CoQ, which is a hydrophobic coenzyme that is located within the membrane. The enzyme the catalyses this redox reaction is known as Complex I. Now, CoQ in reduced form will transfer its electron to a hydrophilic enzyme called Cytochrome C, or only CytoC. The enzyme the catalyses this transfer is known as Complex III. Finally, CytoC transfers its electron to oxygen, through the help of Complex IV.
What about FADH?
The process of transfer of electrons from FADH to oxygen is very similar to the one regarding NADH. The difference however is associated with the use on enzyme Complex II. The use of this enzyme will NOT result in the pumping of hydrogen ions across the membrane, meaning that the oxidation of FADH will not pump as much hydrogen ion as of the one of NADH.
segunda-feira, 1 de dezembro de 2014
domingo, 30 de novembro de 2014
Fermentation and Biofuels
Economic and Environmental Impacts of Ethanol Biofuel Production From Plants
Biofuels have attracted a lot of interest as alternative sources of energy that are considered as more sustainable and "environmentally friendly." In fact, many nations around the world have heavily invested in biofuel production.
Nevertheless, several concerns have arose regarding the use of biofuels. One concern is regarding the amount of corn necessary to produce the ethanol required to replace the currently source of energy. The production of ethanol from corns also increases the eutrophication and pollution of groundwater and aquatic ecosystems. Leading to an increase in greenhouse gases, even though it is carbon dioxide neutral.
There are other types of plants other than corn that can produce biofuels as well. How do they do compared to corn?
Sugarcane
sugarcane produces ethanol in a way more efficient way than corns. Not only, it produces way more ethanol than corns, it actually doens't emit as much greenhouses gases as corns. Nevertheless, it does effect the environment once, in order to produce sugarcane fields, a lot of the rain forest must be cut down. Leading to a increase in impact on biodevisity and carbon dioxide emission to the atmosphere.
Wheat
Although Canada has a smaller biofuel industry than countries like USA and Brazil, we still have several plants that can be use in the production of ethanol. In West Canada, the plant of interest is wheat. The grain of wheat is rich in starch and it has been suggested that genetic engineering could further boost starch content is these grains, making wheat a very efficient source of biofuel.
The Biological Process of Ethanol Production From Fermentation of Plant Material
Bioethanol is produced when carbohydrates are fermented by yeast (a single-celled fungus). A very famous type of yeast is S. cervisiae, which has been used by humans for years to produce ethanol by fermentation.
The start point for ethanol production process is to provide the yeast with a source of carbohydrates, such as corn, sugarcane, and wheat. The yeast uses it as source of energy and carbon. It will break down simple sugars first (such as glucose) and it will generate ethanol and carbon dioxide.
Biofuels have attracted a lot of interest as alternative sources of energy that are considered as more sustainable and "environmentally friendly." In fact, many nations around the world have heavily invested in biofuel production.
Nevertheless, several concerns have arose regarding the use of biofuels. One concern is regarding the amount of corn necessary to produce the ethanol required to replace the currently source of energy. The production of ethanol from corns also increases the eutrophication and pollution of groundwater and aquatic ecosystems. Leading to an increase in greenhouse gases, even though it is carbon dioxide neutral.
There are other types of plants other than corn that can produce biofuels as well. How do they do compared to corn?
Sugarcane
sugarcane produces ethanol in a way more efficient way than corns. Not only, it produces way more ethanol than corns, it actually doens't emit as much greenhouses gases as corns. Nevertheless, it does effect the environment once, in order to produce sugarcane fields, a lot of the rain forest must be cut down. Leading to a increase in impact on biodevisity and carbon dioxide emission to the atmosphere.
Wheat
Although Canada has a smaller biofuel industry than countries like USA and Brazil, we still have several plants that can be use in the production of ethanol. In West Canada, the plant of interest is wheat. The grain of wheat is rich in starch and it has been suggested that genetic engineering could further boost starch content is these grains, making wheat a very efficient source of biofuel.
The Biological Process of Ethanol Production From Fermentation of Plant Material
Bioethanol is produced when carbohydrates are fermented by yeast (a single-celled fungus). A very famous type of yeast is S. cervisiae, which has been used by humans for years to produce ethanol by fermentation.
The start point for ethanol production process is to provide the yeast with a source of carbohydrates, such as corn, sugarcane, and wheat. The yeast uses it as source of energy and carbon. It will break down simple sugars first (such as glucose) and it will generate ethanol and carbon dioxide.
sábado, 29 de novembro de 2014
Regulation of Enzymes
Allosteric Enzymes are a special type of enzymes that change their shape when bound with an activator or inhibitor. The change in shape changes the activation site, which effects on the efficiency of the enzyme. This is important in order to regulate the production of ATP depending on the present requirement of the cell.
Krebs Cycle
We have learnt previously about the pyruvate molecule, and how two of those are produced for every glucose molecule. Now we will see in a greater detail what happens after.
Fermentation
This is anaerobic process, meaning that no oxygen is required for it to happen. Fermentation can lead to lactic acid or ethanol and carbon dioxide + 2 ATP molecules. Some organisms are able to only live from fermentation.
We humans cannot live only through fermentation. This process is just temporary for us, only when oxygen is not available. NADH is not recycled because, even though during the process of fermentation NAD is back, when oxygen is once again available, lactic acid is transformed in pyruvate, and NAD transforms into NADH.
As well as glycolysis, fermentation occurs in the cytoplasm.
The Bridge Reaction
After glycolysis is done, and if there is oxygen available, then pyruvate is transported into the mitochondrial matrix and will reacted with coenzyme A, which will transform it into acetyl-CoA. The result also include the formation of carbon dioxide and 2 NADH (one for each pyruvate.)
This reaction is called the bridge reaction because it links glycolysis to the Krebs cycle (citric acid cycle).
The Krebs Cycle
 The Krebs cycle is a series of 8 connected reactions. The first reaction includes the reaction of acetyl-CoA with oxaloacetate, interesting enough the eight reaction will form this reactant, hence the name "cycle"
The Krebs cycle is a series of 8 connected reactions. The first reaction includes the reaction of acetyl-CoA with oxaloacetate, interesting enough the eight reaction will form this reactant, hence the name "cycle"
Just like glycolysis we will cite the some wonders of this cycle:
Fermentation
This is anaerobic process, meaning that no oxygen is required for it to happen. Fermentation can lead to lactic acid or ethanol and carbon dioxide + 2 ATP molecules. Some organisms are able to only live from fermentation.
We humans cannot live only through fermentation. This process is just temporary for us, only when oxygen is not available. NADH is not recycled because, even though during the process of fermentation NAD is back, when oxygen is once again available, lactic acid is transformed in pyruvate, and NAD transforms into NADH.
As well as glycolysis, fermentation occurs in the cytoplasm.
The Bridge Reaction
After glycolysis is done, and if there is oxygen available, then pyruvate is transported into the mitochondrial matrix and will reacted with coenzyme A, which will transform it into acetyl-CoA. The result also include the formation of carbon dioxide and 2 NADH (one for each pyruvate.)
This reaction is called the bridge reaction because it links glycolysis to the Krebs cycle (citric acid cycle).
The Krebs Cycle
 The Krebs cycle is a series of 8 connected reactions. The first reaction includes the reaction of acetyl-CoA with oxaloacetate, interesting enough the eight reaction will form this reactant, hence the name "cycle"
The Krebs cycle is a series of 8 connected reactions. The first reaction includes the reaction of acetyl-CoA with oxaloacetate, interesting enough the eight reaction will form this reactant, hence the name "cycle"Just like glycolysis we will cite the some wonders of this cycle:
- It consists of 8 connected reaction, which some of them are even coupled reactions.
- It is a cycle because one of the reactants of the first reaction is formed in the last reaction.
- Every reaction is exergonic.
- Every reactions has its own enzyme.
- It forms 2 ATP molecules (1 per pyruvate)
- It forms 6 NADH and 2 FADH for both pyruvates. Both have a lot of energy in their bonds.
The formation of NADH and FADH leads to this idea that they must be recycled. Therefore, it is important the presence of oxygen, because it allow for the oxidative phosphorylation to happen, which we will discuss in the next posts.
Glycolysis
After every meal we have, the food is broken down in our stomachs into small molecules. The most important molecule is known to be glucose. Glucose provides energy for all different kinds of cells and tissues in our body, allowing cellular processes to take place. This is done by the transfer of the potential energy found in the bonds of glucose into the chemical bonds of ATP, through biochemical reactions.
For the next posts, we will have a overlook in these biochemical reaction and their mechanism.
Glycolysis Overview
Glycolysis is a series of 10 connected reactions that will break down glucose and will result in pyruvate. The point of this post is not trying to get you to memorize, instead we will have a overview in some of the principles of this process, in order to comprehend it better.
The first thing to notice is that several molecules of ADP, ATP, NAD, and NADH are involved with the reactions. Glycolysis will result in the formation of two identical pyruvate molecules. Another thing to notice is that glycolysis is a anerobic process.
The 7 principles of Glycolysis
- Glycolysis is a series of 10 connected reactions. Some of these reactions are coupled.
- During the process of glycolysis, glucose is broken in half, hence its potential energy is rearranged in the bonds.
- Every single reaction is exergonic (obviously). Some will small negative free energy, while others will have large.
- Every reaction have its own enzyme. Interesting enough, cells can actually ihibit or activate them depending on how much ATP they need.
- Glycolysis primes ATP. Which means that it actually uses 2 ATP molecules in order to make 4.
- Glycolysis is anaerobic, but will produce two NADH molecules which will be used later on with their high potential energy levels.
- It produces 2 pyruvate molecules, which are still full of energy and must be reused in another reaction.
What happens after glycolysis
We just talked about the formation of NADH. These molecules come from NAD, and these must me recycled, otherwise, there wouldn't be any more NAD to be used.
Gladly there are several ways to recycle NADH. In order to do so, there must be a oxidizing agent, which can be oxygen (if present), or pyruvate itself. When pyruvate is used, it can either format lactic acid, or ethanol and carbon dioxide.
When the presence of oxygen occurs we will have what we called Oxidative Phosphorylation which we will see in later posts.
Membrane and Transport
The cellular membranes are there to isolate the cell from the outside environment. Nevertheless, cells are under constant conditions such as:
Therefore, cells must have a selective barrier.
This selectiveness is due to the very selective permeability of the phospholipid bilayer. It allows small uncharged molecules, small hydrophobic molecules to pass freely, but won't let any charged or large molecules to go through easily.
There are two main type of transport across cellular membranes: (1) diffusion and (2) active transport.
Occurs when molecules are able to pass through the membrane with no help from the any protein. Oone example would be the transportaion of oxygen. Oxygen is not polar or charged, so therefore can pass through. It is also found in greater quantities outside the cell, then inside of it. The membrane also lets the passage of water and carbon dioxide pass thorough, even though they are polar, but they are also very small.
Facilitated Diffusion
The diffusion of some molecules, as sodium ions requires a protein to facilitate the process. This is because these molecule can be charged or too big. Therefore they will need proteins to work as gates so they can pass through the hydrophobic membrane.
Water can also be transported through facilitated transport. Aquaporins are water channels that increase the rate of water movement!
Primary Active Transport
Some molecules don't have the chance to be pass through by passive transportantion, simply because they are already found in higher concetration inside the cell (or outside if the cell is trying to exit it). Nevertheless, these molecules will need energy in order to be transported. In primary active transport the enrgy comes from other reactions such as the hydrolysis of ATP(so it basecally says that PAT uses ATP).
Secondary Active Transport
This mode of transport acquires energy from other transportation process, which must be passive since it provides energy for the other type.
There are two types: Symport (when the two transportations are in the same direction) and antiport (when both are going in opposite directions.)

- Cells live in dynamic environments, where conditions are constantly fluctuating;
- Cells must maintain homoeostasis;
- Cells must regulate the concentration of molecules inside them;
- Cells must regulate the transport of molecules across membranes;
Therefore, cells must have a selective barrier.
This selectiveness is due to the very selective permeability of the phospholipid bilayer. It allows small uncharged molecules, small hydrophobic molecules to pass freely, but won't let any charged or large molecules to go through easily.
There are two main type of transport across cellular membranes: (1) diffusion and (2) active transport.
- Diffusion is also known as passive transport. It does not require any source of energy because the molecules are moving down in their concentration gradient (high to low). It is divided into two types: simple diffusion and facilitated transport.
- Active transport requires energy because the molecules are being transported against its concentration gradient. The source of energy comes from a reaction or process that is coupled with the transport so that there is a negative free energy overall, and therefore, is spontaneous.It is divided into primary transport and secondary transport.
Occurs when molecules are able to pass through the membrane with no help from the any protein. Oone example would be the transportaion of oxygen. Oxygen is not polar or charged, so therefore can pass through. It is also found in greater quantities outside the cell, then inside of it. The membrane also lets the passage of water and carbon dioxide pass thorough, even though they are polar, but they are also very small.
Facilitated Diffusion
The diffusion of some molecules, as sodium ions requires a protein to facilitate the process. This is because these molecule can be charged or too big. Therefore they will need proteins to work as gates so they can pass through the hydrophobic membrane.
Water can also be transported through facilitated transport. Aquaporins are water channels that increase the rate of water movement!
Primary Active Transport
Some molecules don't have the chance to be pass through by passive transportantion, simply because they are already found in higher concetration inside the cell (or outside if the cell is trying to exit it). Nevertheless, these molecules will need energy in order to be transported. In primary active transport the enrgy comes from other reactions such as the hydrolysis of ATP(so it basecally says that PAT uses ATP).
Secondary Active Transport
This mode of transport acquires energy from other transportation process, which must be passive since it provides energy for the other type.
There are two types: Symport (when the two transportations are in the same direction) and antiport (when both are going in opposite directions.)

Energy at Membranes
Cells need to isolate their molecules, organelles and processes that are occurring within them. That is why, cells have thin membranes that surround it. These membranes are not only use to separate compartments, they play a very important role in energy processing.
Cells have the tendency to separate a high energy outside from a lower energy inside. This is done by the difference in electrical charges associated with certain ions and differences in the concentration of certain molecules.
A good example of these difference would be the hydrogen ion. They are usually found in greater amounts on the outside of the cell. Therefore, there is a greater positive charge outside than inside. This difference in charges and concentration will cause the hydrogen ions to push themselves into the cell. Now, this process is very important because it does generate ATP, once it passes by a "turbine" within the membrane, generating a useful work.The sum of the concentration push and electrical push is known to be electrochemical gradient.
The cellular membrane is formed primarily by phospholipids, these make the main structure of this membrane in what is called bilayer. These phospholipids are divided into two parts: The head group which is hydrophilic, formed by several oxygen atoms and one or more nitrogen atoms (polar bonds). The head group likes to interact with water via dipole-dipole interactions, hydrogen bonds, and even ion-dipole interactions. This allows the build up of sterols, which will provide the membrane rigidity and integrity during temperature changes.

The second part of the phospholipids stay in the centre of the membrane. They do not contain any oxygen nor nitrogen atoms, thus, they are hydrophobic, and will have hydrophobic interactions with each other. There are two type in the hydrophobic portion: (1)saturated and (2)unsaturated portions.
This hydrophobic portion of the membrane will keep H ions out or in, creating a electrochemical gradient. That is why there will be proteins built into the membrane. These proteins will not only work as gates for this chemical to pass through the membrane, but they will also play a very important role in production of energy and sensory information.
Membranes keep a constant state of flux, so homoeostasis can be constantly achieved regardless of change in environment.
Summary: The basic structure a cellular membrane is demonstrated by the the Fluid Mosaic Model, which consists of a lipid bilayer which has a hydrophobic centre and hydrophilic surfaces. proteins may be attached to the surface of the lipid bilayer or inserted into or across it. Besides providing physical separation cellular membranes are very important for energy processes in the cell. If the concentration gradient is formed which stores free energy. If the number of cation or anions on one side of the membrane is greater than the number of counter-ions, then an electrical gradient is also formed which stores free energy. Often the two together to form an electrochemical gradient.
Cells have the tendency to separate a high energy outside from a lower energy inside. This is done by the difference in electrical charges associated with certain ions and differences in the concentration of certain molecules.
A good example of these difference would be the hydrogen ion. They are usually found in greater amounts on the outside of the cell. Therefore, there is a greater positive charge outside than inside. This difference in charges and concentration will cause the hydrogen ions to push themselves into the cell. Now, this process is very important because it does generate ATP, once it passes by a "turbine" within the membrane, generating a useful work.The sum of the concentration push and electrical push is known to be electrochemical gradient.
The cellular membrane is formed primarily by phospholipids, these make the main structure of this membrane in what is called bilayer. These phospholipids are divided into two parts: The head group which is hydrophilic, formed by several oxygen atoms and one or more nitrogen atoms (polar bonds). The head group likes to interact with water via dipole-dipole interactions, hydrogen bonds, and even ion-dipole interactions. This allows the build up of sterols, which will provide the membrane rigidity and integrity during temperature changes.

The second part of the phospholipids stay in the centre of the membrane. They do not contain any oxygen nor nitrogen atoms, thus, they are hydrophobic, and will have hydrophobic interactions with each other. There are two type in the hydrophobic portion: (1)saturated and (2)unsaturated portions.
- Saturated layer are packed more tightly and make the membrane less fluid
- Unsaturated layer are less packed and make the membrane less packed, making it more fluid.
This hydrophobic portion of the membrane will keep H ions out or in, creating a electrochemical gradient. That is why there will be proteins built into the membrane. These proteins will not only work as gates for this chemical to pass through the membrane, but they will also play a very important role in production of energy and sensory information.
Membranes keep a constant state of flux, so homoeostasis can be constantly achieved regardless of change in environment.
Summary: The basic structure a cellular membrane is demonstrated by the the Fluid Mosaic Model, which consists of a lipid bilayer which has a hydrophobic centre and hydrophilic surfaces. proteins may be attached to the surface of the lipid bilayer or inserted into or across it. Besides providing physical separation cellular membranes are very important for energy processes in the cell. If the concentration gradient is formed which stores free energy. If the number of cation or anions on one side of the membrane is greater than the number of counter-ions, then an electrical gradient is also formed which stores free energy. Often the two together to form an electrochemical gradient.
Classification of Organisms
In order to comprehend a little better the vast diversity of organisms in nature, we have looked for classifications systems. Traditionally, animals have been classified based on the morphology (shape and structure, or simply "how they look like"). This led to the creation of the 5 kingdoms. Nevertheless, a better and more accurate system of classification is used today. Now, organisms are based on their:
Classification based on phylogenetics
Phylogenetics is just a fancy word for molecular information, specifically ribosomal RNA gene sequence. Interesting enough, each organism has a specific sequence. Therefore, by comparing the sequence of genes in different organism we can see how similar they are from each other. This led us to assume that all organisms could be grouped into three different domains: (1) eukaryota, (2) bacteria, and (3) archaea.
Classification based on cellular complexity:
When classifying animals, we must analyse in detail the complexity within the organism. Therefore, we will divide the organisms based on the complexity of their cells. This leads us to two types of cell: (1) Prokaryotic, and (2) Eukaryotes.
 There are two very important misleading ideas in this topic:
There are two very important misleading ideas in this topic:
- The first one is regarding the uni-cellular organisms. These are not necessarily bacteria or archea. Some are considered to be eukaryota.
- The second one is the prokaryotic organisms are not always similar to each other, we have already seem that archea is very similar to eukaryota and not to bacteria.
Interesting enough, we can think about relationships between prokaryotes and eukaryotes. Think about your mouth, there are billions of prokaryotes organisms living there, in a symbiotic relationship.
There is a theory called endosymbiotic theory. It suggests that some organelles in eukaryotic cells such as mitcochondria and chloroplasts were originated from prokaryotes! And there is some evidence for this statement: There are many symbiotic and endosymbiotic association today, these organelles have their own DNA and divid by binary fission (just like prokaryotes), and their ribossomes are more similar to prokaryotes than to eukaryotes.
Classification based on energy and carbon source
Organisms that acquire the energy from the sun are said to me phototroph, other organisms which have their energy coming from the chemical bonds of molecules are said to be chemotroph. We also know that there are two types of chemotrophs: those who get energy from organic chemical compounds (chemoorganotrophs), and those who get energy from non-organic chemical compounds (chemolithotrophs).
Now we will learn that the source of carbon can actually influence on the classification of organisms.
Those organisms that have their carbon source from carbon dioxide are said to be autotrophs, and those who get from organic compounds are said to be heterotroph. When naming, we put both energy and carbon source names into one.
For example, plants are said to be photo-auto-trophs, because they get their energy from the sun and the carbon dioxide is their source of carbon. Us, humans we are chemo-organo-hetero-trophs. Can you say why?
Summary: Phylogenetic classification of organisms based on rRNA sequences has led to classification into theree domains in life - bacteria, archae, and eukaryota. Although archae are prokaryotic cells, they are more closely genetically related to eukaryote than bacteria as is shown by the examples in the text. The classification of organisms based on energy source (photo- or chemo-) and carbon source (auto- or hetero-) lead to six different classes.
- Phylogenetics;
- Cellular complexity;
- Energy Source;
- Carbon Source.
Classification based on phylogenetics
Phylogenetics is just a fancy word for molecular information, specifically ribosomal RNA gene sequence. Interesting enough, each organism has a specific sequence. Therefore, by comparing the sequence of genes in different organism we can see how similar they are from each other. This led us to assume that all organisms could be grouped into three different domains: (1) eukaryota, (2) bacteria, and (3) archaea.
- Eukaryota include organisms that are relative complex compared to their other domains. They have bigger and more complex cells and membrane bound organelles also.
- Bacteria are found in several forms and shapes. These are single-celled organisms that have a great diversity on ways to metabolise energy. Bacteria also do not have membrane bound organelles like eukaryota organisms.
- Archae organisms are very similar to bacteria, so similar that they were once formerly considered to be bacteria! Nevertheless, by comparing rRNA, it was concluded that they belonged to different domains. These organism have very usual shapes, and live in very extreme environments, such as high salt concentration, acidic water, or thermal hot springs.
Classification based on cellular complexity:
When classifying animals, we must analyse in detail the complexity within the organism. Therefore, we will divide the organisms based on the complexity of their cells. This leads us to two types of cell: (1) Prokaryotic, and (2) Eukaryotes.
- Prokaryotic cells are small and simple when comparing to eukaryotes, therefore, having a higher surface-to-volume ratio. The do not have membrane bound molecules, and its genetic information is all around the place within the cell. By being small though, prokaryotic cells don't need much in order to maintain themselves.
- Eukaryotic cells are way bigger and more complex. They do have membrane bound molecules, and their genetic material are located in a membrane bounded nucleus. These membrane bound molecules are very important because they allow specific compartments to carry out specific function like energy production. This allows this big cells to maintain themselves.
 There are two very important misleading ideas in this topic:
There are two very important misleading ideas in this topic:- The first one is regarding the uni-cellular organisms. These are not necessarily bacteria or archea. Some are considered to be eukaryota.
- The second one is the prokaryotic organisms are not always similar to each other, we have already seem that archea is very similar to eukaryota and not to bacteria.
Interesting enough, we can think about relationships between prokaryotes and eukaryotes. Think about your mouth, there are billions of prokaryotes organisms living there, in a symbiotic relationship.
There is a theory called endosymbiotic theory. It suggests that some organelles in eukaryotic cells such as mitcochondria and chloroplasts were originated from prokaryotes! And there is some evidence for this statement: There are many symbiotic and endosymbiotic association today, these organelles have their own DNA and divid by binary fission (just like prokaryotes), and their ribossomes are more similar to prokaryotes than to eukaryotes.
Classification based on energy and carbon source
Organisms that acquire the energy from the sun are said to me phototroph, other organisms which have their energy coming from the chemical bonds of molecules are said to be chemotroph. We also know that there are two types of chemotrophs: those who get energy from organic chemical compounds (chemoorganotrophs), and those who get energy from non-organic chemical compounds (chemolithotrophs).
Now we will learn that the source of carbon can actually influence on the classification of organisms.
Those organisms that have their carbon source from carbon dioxide are said to be autotrophs, and those who get from organic compounds are said to be heterotroph. When naming, we put both energy and carbon source names into one.
For example, plants are said to be photo-auto-trophs, because they get their energy from the sun and the carbon dioxide is their source of carbon. Us, humans we are chemo-organo-hetero-trophs. Can you say why?
Summary: Phylogenetic classification of organisms based on rRNA sequences has led to classification into theree domains in life - bacteria, archae, and eukaryota. Although archae are prokaryotic cells, they are more closely genetically related to eukaryote than bacteria as is shown by the examples in the text. The classification of organisms based on energy source (photo- or chemo-) and carbon source (auto- or hetero-) lead to six different classes.
terça-feira, 18 de fevereiro de 2014
Unit Circle
 In mathematics, a unit circle is a circle with a radius
of one. The unit circle can be used in trigonometry, when placed in a Cartesian
coordinate system, so that its center is at the origin (0,0).
In mathematics, a unit circle is a circle with a radius
of one. The unit circle can be used in trigonometry, when placed in a Cartesian
coordinate system, so that its center is at the origin (0,0).
Now, if we have (x,y) as a point
on the unit circle, and knowing that the radius of the circle is equal to one,
we may form a triangle and therefore acquire a equation, called the equation of
unit circle: x2 + y2
= 1.
Now, let's get more into trigonometric function of
the unit circle. If we have a point on the unit circle (x, y) and we form a
triangle with it, a angle "t" will be formed.
Do you remember the definitions of cosine and sine?
Well, if you do, you will know that, considering the radius (hypotenuse) being
equal one, cos(t) = x and sin(t) = y. We also already know the equation of the
unit circle (x2
+ y2 = 1). Now let's sub the x and y with cos and sin. This will
result in cos2(t) + sin2(t) = 1
Knowing this equation, it is easy to determine
points and angles on the unit circle. However, to be more practical you must
memorise the unit circle, not only the angles, but the radians.
segunda-feira, 17 de fevereiro de 2014
Angles
Co-Terminal
Angles
 Co-terminal angles are angles
with initial side on the positive x-axis, also called standard position, that have a common terminal side. For example 30o,
-330o and 390o are all co-terminal. To find a angle's co-terminal angle, you just
need to add or subtract 36o degrees, if the angle is measured in degrees,
or 2 pi if the angle is measured in
radians.
Co-terminal angles are angles
with initial side on the positive x-axis, also called standard position, that have a common terminal side. For example 30o,
-330o and 390o are all co-terminal. To find a angle's co-terminal angle, you just
need to add or subtract 36o degrees, if the angle is measured in degrees,
or 2 pi if the angle is measured in
radians.
Ex: Find
a positive and a negative angle coterminal with a 55° angle.
55° – 360° = –305°
55° + 360° = 415°
A –305° angle and a 415°
angle are coterminal with a 55° angle
Principal
Angle
The principal angle is the least
positive angle that a circle can provide. Always count not clock wise,
therefore the principle angle is between 0 and 360. or 0 and 2 pi.
Reference
Angle
The angle formed between the
terminal arm and the x-axis. Also called the bow tie rule.
Radian
The radian, until 1995, used to be the standard unit of angular measurement.
Nevertheless, this unit of measurement is often used in many fields of
mathematics. Even though its unit symbol
may be "radian" or "rad," it is usually omitted. Therefore,
radian is called a dimensionless quantity.
When you need to convert radians into degrees, you must multiply the value you have by 180 and the divide the result by pi. If you have your values and degrees, and wants to convert to radians, take your values and multiply by pi, then divide by 180.
The radian measure was first used
in opposed to the degree of an angle by Roger
Cotes in 1714. However, the term radian was only used in print form on 5th
June of 1873, in examination questions set by James Thomson, at Queen's College, Belfast.
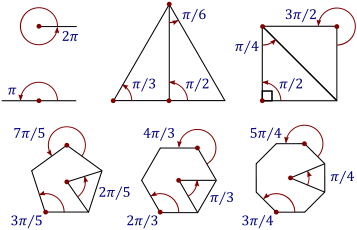 It is important to understand the concepts of radian measures because, in calculus, angles are universally measured in this unit. The
reason for this is because it can lead to a more elegant formulation of a number of important results than
degrees of angles would, since radian is a pure measure based on the radius of
the circle. When the results in analysis involves trigonometric functions for
example, radians are used in order to have the results expressed in a more
elegant and simple way. To conclude the thought: "Degrees are more practical,
but radians are more elegant and mathematically easier."
It is important to understand the concepts of radian measures because, in calculus, angles are universally measured in this unit. The
reason for this is because it can lead to a more elegant formulation of a number of important results than
degrees of angles would, since radian is a pure measure based on the radius of
the circle. When the results in analysis involves trigonometric functions for
example, radians are used in order to have the results expressed in a more
elegant and simple way. To conclude the thought: "Degrees are more practical,
but radians are more elegant and mathematically easier." sexta-feira, 24 de janeiro de 2014
Eletricalchemical Changes
Exchange of Electrons.
Two terms: Oxidation and Reduction.
Oxidation: - Any reaction with oxygen;
-Loss of Electron.
Reduction: - Any reaction that produced metal from ores;
- Gain of Electrons.
REDOX reaction: There is gaining and losing of electrons.
Oxidizing agent (OA) is defined as the one entity that will donate electrons, therefore promoting oxidation on the other entity.
Reduction agent (RA) is defined as the one entity that will receive that electron, therefore promoting the other entity.
DOUBLE REPLACEMENT, OR ACID AND BASE REACTIONS ARE REDOX
quinta-feira, 23 de janeiro de 2014
Thermal Stability
Thermal stability is the tendency of a compound to resist decomposition when heated. Which means that a substance that has the greates decomposition molar enthapy is the one with greates thermal stability. Remember that IF MORE NERGY IS RELEASE WHEN A COMPOUND IS FORMED, THAN IT TAKES MORE ENERGY TO DECOMPOSE IT.
Hess' Law
When you leave you house to go the gym, you may take root 1, or maybe you can take root 2. It does not matter how you get to the gym, as long as you do. Same applies to chemical reaction and enthalpy.
For hess' law, you must know to things:
- Whenever you switch products with reactants and vice versa, you must change your enthalpy sign and well.
-If you multiply the coefficient of the equation, you multiply the enthalpy change value as well.
Hess' law modified
Hess determined that you can actually find the enthalpy change of a reaction, by using the molar enthalpy of the components of the reaction.
When using the method, remember that:
- A balanced equation is necessary;
- Molar enthalpy of formation of elements are equal to 0
- Water vapour is always produces, unless we are talking about photosynthesis, cellular respiration and closed system combustion. Which in the case, liquid water will be formed.
r Ho
=
f Ho
pr Σoducts
f Ho Σ reactant
Calorimetry
Calorimetry is a way scientific expriment to figure the energy released or absorbed by a reaction. In calaorimetry, we use a calorimeter, that is composed by a system (chemical reaction) and surroundings (water or something like water). When the reaction measures a made. and the asusmpition the THERMAL ENERGY CHANGE IS EQUAL TO THE CHANGE IN ENTHALPY, SINCE THE ENERGY LOST OR GAINED BY THE SYSTEM IS EQUAL TO THE ENERGY GAINED OR LOST BY THE SURROUNDINGS.
Don;t forget that when the question gives you the mass of the calorimeter, and its a metal. You must account it as one of the surroundings, which means that not only water is present.
Don;t forget that when the question gives you the mass of the calorimeter, and its a metal. You must account it as one of the surroundings, which means that not only water is present.
Heat Transfer and Calorimetry
Kinetic energy and Potential energy are actually related! They also have specific calculation for each one of them.
Kinetic energy is related to temperature. Therefore, Ek is related to thermal changes. To figure out what is the thermal change in a substance, consequently Ek.
Q = mc^t
Q means thermal change
M means mass
c mens specific heat capacity
^t means the change in temperature.
When we are talking about potential energy, we talk about enthalpy. Enthalpy is the total energy of a substance so Ek and Ep. When we say that there is a change in enthalpy, we talk about the difference between the Ep of the products and reactants. REMEMBER THAT CHANGE IN ENTHALPY DOES NOT HAVE ANYTHING TO DO WITH CHANGE IN TEMPERATURE.
Molar change in enthalpy - Change in the Ep per mole of a substance.
To figure the Enthalpy change, we always look at the calculalio ^^H= n^^Hm
^^^H means enthalpy change
n means moles
^^Hm means molar enthalpy change
The molar enthalpy of FORMATION will be listed on you r data booklet, The molar enthapy of DECOMPOSITION is the same of the formation, but with a reversed sign. For the other reaction, like combustion, it will be said in the question, or you will have to figured it out. The molar enthalpy of elements is 0.
Thermochemical Changes - Intro
Energy
The scientific definition of energy describes it as the ability to do work. Without energy, no reactions could occur, therefore, our lives would be impossible to continue. ALL ENERGY ORIGINATES FROM THE SUN!
In this unit we will study the two types of energy that are extremely related to chemical changes and reactions: Kinetic and Potential Energy.
1) Kinetic:
It is an energy that is related to movement, and in the case of chemistry, we will be talking about the movement of particles and molecules. Therefore, we will understand that when particles, or molecules, are moving faster, their kinetic energy is increasing. When moving slower, their kinetic energy will decrease.
Kinetic Energy is very related to temperature. When a substance increases its temperature, it means that the particles are moving fast, therefore, we must have a large kinetic energy. The opposite occurs, when the temperature of a substance decreases.
As we know from previous studies, temperature gives us clues of heat. What is heat? Heat is the transfer of thermal energy between two substance or objects.
2) Potential:
This energy is related to the bonds of molecules in a substance, when they are formed or broken down, potential energy is changed. In this units, we will focus on the potential energy in the intra molecular bonds, which are of course the bonds between atoms that form a molecule. When this bonds are broken down, or formed, that means that a chemical change, or we can say, a chemical reaction is occurring.
There are two types of chemical reactions: Endothermic and Exothermic Reactions.
Endothermic reactions are chemical reaction which energy is required in other to occur. The best example of endothermic reaction is PHOTOSYNTHESIS. Exothermic reaction in another hand is the chemical reaction that releases energy. The best example of exothermic reaction is CELLULAR RESPIRATION.
Assinar:
Comentários (Atom)



