- Write the unbalanced equation that show the formulas of the given reactants and products.
- Balance the atoms other than oxygen and hydrogen.
- Balance the atoms of Oxygen by adding water.
- Balance the hydrogen atoms by adding hydrogen ions. (At this point, if we are dealing with an acidic solution, balance the charges by adding the electrons.)
- If we are dealing with a basic solution: Add to both sides, the same number of hydroxide ions as the number or hydrogen ions present.
- Add the hydrogen and hydroxide ions on the same side to form water.
- Cancel and water molecule present on both sides of the reaction.
- Balance the charges by adding the electrons.
sexta-feira, 4 de outubro de 2013
Balancing Equations - Acidic and Basic Solutions
When we take in account reactions that are acidic or basic, we must consider the hydrogen ion and hydroxide ion. The overall approach regarding balancing these equation is very similar to a simple reaction, however, few more steps are required.
quarta-feira, 2 de outubro de 2013
Redox Reactions
Law of Conservation of Energy:
No energy is created, nor destroyed, it is transformed or transferred.
Yes, and in a chemical reaction? aren't there like several exchange of electrons?
If each atom or ion of a chemical reaction is oxidized, that another must be reduced. When this happens we have a redox reaction.
Redox reactions are not a type of reaction, like formation, or decomposition.
Actually, all the types of reactions may be redox reactions.
To understand this concept better let's use this simple single-replacement reaction:
Zn(s)+CuSO4(aq)=Cu(s)+ZnSO4(aq)
Lets take away the spectators, which, in this case are the SO4 ions.
You obtain
Zn(s) + Cu+2(aq) = Cu(s)+Zn+2
As you see, Zinc lost electrons, it became positive, thus got oxidized. Copper, gained two electrons, since it was a cation and became a single atom. Therefore it was reduced.
Well, we know that the electrons ran away from Zn to Cu, so what do we do about it?
Zinc is called the reducing agent, the one that donated electron, and the one the underwent oxidation, while copper is the oxidizing agent, the one that accepeted electrons, undergoes reduction.
No energy is created, nor destroyed, it is transformed or transferred.
Yes, and in a chemical reaction? aren't there like several exchange of electrons?
If each atom or ion of a chemical reaction is oxidized, that another must be reduced. When this happens we have a redox reaction.
Redox reactions are not a type of reaction, like formation, or decomposition.
Actually, all the types of reactions may be redox reactions.
To understand this concept better let's use this simple single-replacement reaction:
Zn(s)+CuSO4(aq)=Cu(s)+ZnSO4(aq)
Lets take away the spectators, which, in this case are the SO4 ions.
You obtain
Zn(s) + Cu+2(aq) = Cu(s)+Zn+2
As you see, Zinc lost electrons, it became positive, thus got oxidized. Copper, gained two electrons, since it was a cation and became a single atom. Therefore it was reduced.
Well, we know that the electrons ran away from Zn to Cu, so what do we do about it?
Zinc is called the reducing agent, the one that donated electron, and the one the underwent oxidation, while copper is the oxidizing agent, the one that accepeted electrons, undergoes reduction.
Reduction
So, do you remember that little law of thermodynamics? What was it?
Energy is never created, or destroyed, it is always transformed...
...or transferred.
When one atom, ion, or molecule, loses a electron, this little particle cannot just run around the environment. He needs a place to stay, a house. And what kind of house am i talking about?
Another atom, ion, or molecule.
Yes, they must gain the electrons that were lost by other elements that got oxidated.
If oxidation is the loss of electrons, than, the gain of them is called reduction.
Energy is never created, or destroyed, it is always transformed...
...or transferred.
When one atom, ion, or molecule, loses a electron, this little particle cannot just run around the environment. He needs a place to stay, a house. And what kind of house am i talking about?
Another atom, ion, or molecule.
Yes, they must gain the electrons that were lost by other elements that got oxidated.
If oxidation is the loss of electrons, than, the gain of them is called reduction.
Oxidation
Okay! So, now let's look a little bit forward onto chemical reaction.
Our first topic is Oxidation.
Back in the days, chemists believed that oxidation was any reaction that occurred between any atom or compound with a molecular oxygen.
However, after many and many years of study and analysis, it was learned that same reaction between atoms or compounds with other elements have similarities with those reaction involving oxygen.
So the scientist came out with another definition for oxidation, which is the loss of electrons by an atom or an ion.
For example, when Mg(s) reacts with Cl2(g), our little buddy Magnesium atom loses electrons, becoming a cation. We can say, therefore, that Magnesium got oxidised.
Our first topic is Oxidation.
Back in the days, chemists believed that oxidation was any reaction that occurred between any atom or compound with a molecular oxygen.
However, after many and many years of study and analysis, it was learned that same reaction between atoms or compounds with other elements have similarities with those reaction involving oxygen.
So the scientist came out with another definition for oxidation, which is the loss of electrons by an atom or an ion.
For example, when Mg(s) reacts with Cl2(g), our little buddy Magnesium atom loses electrons, becoming a cation. We can say, therefore, that Magnesium got oxidised.
domingo, 29 de setembro de 2013
Hess' Law
Let's study a little bit of history:
In 1830, a Russian chemist and doctor known and Germain Henri Hess, formulate a law that it is still used today. It is called the Hess law.
According to Hess, the enthalpy change of a chemical process, only depend on the initial and final state, not on its pathway. The enthalpy change of the overall process is the sum of the enthalpy change of its individual steps.
In 1830, a Russian chemist and doctor known and Germain Henri Hess, formulate a law that it is still used today. It is called the Hess law.
According to Hess, the enthalpy change of a chemical process, only depend on the initial and final state, not on its pathway. The enthalpy change of the overall process is the sum of the enthalpy change of its individual steps.
Methods of Communicating Enthalpy Changes
Chemists have different ways to express the enthalpy change of a reaction. Which would be?
- Stating molar enthalpy of a specific reactant of the reaction;
- Stating the enthalpy change for a balanced equation.
- Including the enthalpy change as a part of the equation, being reactant or product, depending on the type of the reaction;
- Representing by a diagram.
They will provide us a better understanding on the topic.
Which is?
The change in the energy of substances after a reaction occur.
Which ones of these four methods are we can trust the most?
The first three ones, since they are empirical description. The fourth one, for being a diagram, is more of a theoretical description.
Let's talk about Method 1:
Well, the molar enthalpy is the measure of released or absorbed energy from or to a substance per mole. The substance and the reaction must be specified.
Do we have standards: Yes, we do. Standard molar enthalpy are expressed with a * subscript. It means that it represents the molar enthalpy of substance under ideal conditions.
Method 2:
Just write the enthalpy change next to the balanced equation. Iif we change the coefficient or the order of the equation, then the enthalpy change will also change.
Method 3:In endothermic reactions the energy is on the reactant side. in exothermic reaction the energy is on the product.
Calorimetry II
Alright, but let's think this through... If metals have specific heat capacity, wouldn't my result be affected if I did the procedure in a calorimetry made of Aluminium for example?
Yes, indeed. Calorimeter made of metal have a special attention by the chemists because they know it absorbs or release way too much energy to be ignored.
Thus, in our calculation, we need to use more information that a conventional procedure.
The temperature change of the calorimeter needs to be accounted for. If not stated, you can assume that the temperature change of the metal is the same as that of the water.
Lets not forget about other very special type of calorimetry: The combustion calorimetry.
It is usually done in a bomb calorimeter. With combustion, MASS ID NOT NECESSARY.
Calorimetry
Okay, you are probably thinking about: Where de heck is all those famous experiments used in chemistry?
Well, in thermochemistry we have an experimental method used to measure energy changes. It is called Calorimetry.
Before we start calorimetry, lets recall two very important laws related to this issue:
Calorimetry is the method that use calorimeter which is a device that is used to measure the amount of heat that was released or absorbed through a chemical reaction that occurred within it.
IDEALLY CALORIMETER IS AN ISOLATED SYSTEM! Yes, do not forget that! Because a isolated system means that the environment around the calorimetry DOES NOT affect the final result.
To use calorimeter, it is very important to have some assumption which are:
It is possible to find more currate results with aluminium calorimeters.
The best type is called the bomb calorimeter.
They all work based on the same principles.
Well, in thermochemistry we have an experimental method used to measure energy changes. It is called Calorimetry.
Before we start calorimetry, lets recall two very important laws related to this issue:
- Energy is never created or destroyed, only transformed.
- Heat flow from hotter to cooler substances until they reach the same temperature.
Calorimetry is the method that use calorimeter which is a device that is used to measure the amount of heat that was released or absorbed through a chemical reaction that occurred within it.
IDEALLY CALORIMETER IS AN ISOLATED SYSTEM! Yes, do not forget that! Because a isolated system means that the environment around the calorimetry DOES NOT affect the final result.
To use calorimeter, it is very important to have some assumption which are:
- All energy gained or lost by the calorimeter was gained or lost by the system.
- The calorimeter is completely isolated.
- The liquid in the calorimeter has the same physical proprieties as water.
- The thermal energy gained or lost by the other devices in the calorimeter, such as the container, thermometer, and lid, are too small to be not be ignored.
It is possible to find more currate results with aluminium calorimeters.
The best type is called the bomb calorimeter.
They all work based on the same principles.
Potential Energy - Enthalpy
The second form of energy studied in chemistry is called Potential energy.
Potential energy is commonly called Enthalpy in the chemistry world.
Can we measure potential energy like we measure kinetic energy?
Oh, actually Potential energy is equal to thermal energy, however we cannot measure by using the thermal equation, because it does not require temperature change.
Enthalpy energy is stored in the chemical bonds of molecules within a substance. When these bonds are broken or formed there will be a change in the total enthalpy of the substance.
Therefore we can measure enthalpy change by using the equation:
ΔH = nΔHm
Where ΔH is Enthalpy change (kJ), n (moles) and ΔHm (molar enthalpy).
Whoa whoa, what the heck is molar enthalpy?
Molar enthalpy is the change in the enthalpy when on per mole of substance undergoes a process.
Potential energy is commonly called Enthalpy in the chemistry world.
Can we measure potential energy like we measure kinetic energy?
Oh, actually Potential energy is equal to thermal energy, however we cannot measure by using the thermal equation, because it does not require temperature change.
Enthalpy energy is stored in the chemical bonds of molecules within a substance. When these bonds are broken or formed there will be a change in the total enthalpy of the substance.
Therefore we can measure enthalpy change by using the equation:
ΔH = nΔHm
Where ΔH is Enthalpy change (kJ), n (moles) and ΔHm (molar enthalpy).
Whoa whoa, what the heck is molar enthalpy?
Molar enthalpy is the change in the enthalpy when on per mole of substance undergoes a process.
Kinetic Energy
Kinetic Energy! One of the most abundant forms of energy on earth.
But, wait a minute, shouldn't we be studying this in a physics class?
Nope, actually kinetic energy is very present in chemistry.
Kinetic is the energy of motion. We know that particles are always in movement, thus it will generate energy in the form of movement.
You know how particles' movement influence and is influenced by the temperature temperature change? Well, this is simple to understand if we say that when the temperature increases, the kinetic energy increases as well, and vice versa.
Wait we know what kinetic energy is, but can we measure it?
The answer is no. However, we can measure temperature.
A change in temperature indicates that energy has left or entered a substance. However the amount of energy required to change the temperature of different substance by the same amount, varies with the type of substance.
For example: It actually take 4.19 J of energy to change the temperature of 1 ml of water by one degree, while it takes 0.897 J of energy to change the temperature of 1 g of aluminium by one degree.
These values quoted above are known as the specific heat of a substance. The amount of energy necessary to increase one gram of a substance by one degree Celsius.
It is very important to remember that the state of the substance influences its specific heat capacity.
Okay okay, lets not get out of focus here. We know that we can measure temperature. We also know we can be provided the specific heat capacity of a substance. Okay. Now, can we measure the mass of the substance?
If the answer is yes, we can use these three values and form a equation which will provide us the amount of energy that was exchanged.
Q=mc(tf-ti), where Q represents the amount of energy, m is equal to mass, c is equal to specific heat capacity.
Note that if the temperature change is negative, it will make the Q value be negative. Thus, this equation not only provide the amount of heat, but tells you if the reaction released or absorbed energy.
But, wait a minute, shouldn't we be studying this in a physics class?
Nope, actually kinetic energy is very present in chemistry.
Kinetic is the energy of motion. We know that particles are always in movement, thus it will generate energy in the form of movement.
You know how particles' movement influence and is influenced by the temperature temperature change? Well, this is simple to understand if we say that when the temperature increases, the kinetic energy increases as well, and vice versa.
Wait we know what kinetic energy is, but can we measure it?
The answer is no. However, we can measure temperature.
A change in temperature indicates that energy has left or entered a substance. However the amount of energy required to change the temperature of different substance by the same amount, varies with the type of substance.
For example: It actually take 4.19 J of energy to change the temperature of 1 ml of water by one degree, while it takes 0.897 J of energy to change the temperature of 1 g of aluminium by one degree.
These values quoted above are known as the specific heat of a substance. The amount of energy necessary to increase one gram of a substance by one degree Celsius.
It is very important to remember that the state of the substance influences its specific heat capacity.
Okay okay, lets not get out of focus here. We know that we can measure temperature. We also know we can be provided the specific heat capacity of a substance. Okay. Now, can we measure the mass of the substance?
If the answer is yes, we can use these three values and form a equation which will provide us the amount of energy that was exchanged.
Q=mc(tf-ti), where Q represents the amount of energy, m is equal to mass, c is equal to specific heat capacity.
Note that if the temperature change is negative, it will make the Q value be negative. Thus, this equation not only provide the amount of heat, but tells you if the reaction released or absorbed energy.
Energy
Welcome to the course of Chemistry 30.
Our first topic is ENERGY!!
Energy is essential for life. It provide us motion, fuel, light and many others!
But what is the exactly definition of energy?
Well, some say that energy is the strength necessary to sustain our physical and mental activities. Others, will say is the ability to do work.
However, in this course let's think energy as a amount of power. Power that is essential for life.
There are many forms of energy! Energy of motion, light energy, molecular energy, sound energy, nuclear energy and etc.
It is very important to know that energy originate from the sun!
 Yes, the sun. Our biggest star is bale to convert hydrogen atoms into helium atoms, providing a enormous amount of energy to the earth.
Yes, the sun. Our biggest star is bale to convert hydrogen atoms into helium atoms, providing a enormous amount of energy to the earth.
This energy is converted into chemical energy into the plants by a process called Photosynthesis. This chemical energy is essential for plants to carry its metabolic life.
When animals eat the plants, this energy will now help the new organism to live its metabolic life. And it goes on and on.
When plants or animals consume energy, it is stored in the chemical bonds. When the plant and animal decompose, chemical reaction occur, since bonds are been broken. The energy can either be released or absorbed.
But how sun does not give nuclear energy for example. So how do they all originate there?
Well, to answer this question we have to understand the root of chemistry known as Thermochemistry.
In thermochemistry we study some laws called the laws of conservation of energy.
The first law explains that energy is never created or destroyed, however it is always transformed.
Energy originates from the sun in form of life, however it transforms into all the types of energy we know of.
In chemistry, energy is essential for chemical reactions to happen. Remember this: Bonds to be broken of formed, require energy.
There are many forms of energy! Energy of motion, light energy, molecular energy, sound energy, nuclear energy and etc.
It is very important to know that energy originate from the sun!
This energy is converted into chemical energy into the plants by a process called Photosynthesis. This chemical energy is essential for plants to carry its metabolic life.
When animals eat the plants, this energy will now help the new organism to live its metabolic life. And it goes on and on.
When plants or animals consume energy, it is stored in the chemical bonds. When the plant and animal decompose, chemical reaction occur, since bonds are been broken. The energy can either be released or absorbed.
But how sun does not give nuclear energy for example. So how do they all originate there?
Well, to answer this question we have to understand the root of chemistry known as Thermochemistry.
In thermochemistry we study some laws called the laws of conservation of energy.
The first law explains that energy is never created or destroyed, however it is always transformed.
Energy originates from the sun in form of life, however it transforms into all the types of energy we know of.
In chemistry, energy is essential for chemical reactions to happen. Remember this: Bonds to be broken of formed, require energy.
sábado, 28 de setembro de 2013
Energy and Efficiency
Guys, guys I have a question, what is efficiency?
Well, efficiency can be described in several forms. But when we relate it with energy, we say that efficiency is the ability to produce a desired effect with minimum energy expenditure.
For example, which one uses less energy to cook a potato: A microwave or a normal oven. Of course the microwave uses less energy, thus we say it is more efficient than a oven.
But let's put some math in this definition. Efficiency compares the input and output of energy. The ratio of useful energy produced (energy output) to energy used in its production (energy input), expressed as percentage.
But what it energy output and input?
Energy output is the work done, energy delivered to consumer in usable form. You can find it by calculating Q=mc(tf-ti) or enthalpy change equation.
Energy input is the solar energy, fuel energy, power energy and etc. it is found through Hess' Law.
LAB TIME:
To study energy efficiency, lets determine the efficiency of a barbecue why heat 5.10g of propane, and change the temperature of 250 g of water contained in a 500 g stainless steel pot (c=0.503 j/gxC) from 25C to 75C.
Well first of all lets find the energy output of this problem. By using Hess' Law we know that -236 kj.
Energy output can be figured by thermal energy equation which will give us a value of 65.0 kj
Now you take these two values, divide them and multiple by 100.
Well, efficiency can be described in several forms. But when we relate it with energy, we say that efficiency is the ability to produce a desired effect with minimum energy expenditure.
For example, which one uses less energy to cook a potato: A microwave or a normal oven. Of course the microwave uses less energy, thus we say it is more efficient than a oven.
But let's put some math in this definition. Efficiency compares the input and output of energy. The ratio of useful energy produced (energy output) to energy used in its production (energy input), expressed as percentage.
But what it energy output and input?
Energy output is the work done, energy delivered to consumer in usable form. You can find it by calculating Q=mc(tf-ti) or enthalpy change equation.
Energy input is the solar energy, fuel energy, power energy and etc. it is found through Hess' Law.
LAB TIME:
To study energy efficiency, lets determine the efficiency of a barbecue why heat 5.10g of propane, and change the temperature of 250 g of water contained in a 500 g stainless steel pot (c=0.503 j/gxC) from 25C to 75C.
Well first of all lets find the energy output of this problem. By using Hess' Law we know that -236 kj.
Energy output can be figured by thermal energy equation which will give us a value of 65.0 kj
Now you take these two values, divide them and multiple by 100.
Catalysts
Okay, we've learned that some reactions are very slow. They may take hours, days, weeks, and even years to be completed. But how does it affect the economy?
Yes, economy. We know that many things we use are produced through industrial application. Thus, the slowness of a reaction would make impossible to have any kind of profit in the production of these products.
So how on earth do the guys that work in industry manage to accelerate this reactions?
They use something very important for our daily-basis called catalyst.
But what is catalyst?
Well, catalyst is a substance that increases the rate of a chemical reaction without being consumed by the reaction.
Catalyst is not only important in industry, but it also play a very important role in our body.
And how does it work?
It basically provide an alternative pathway for a reaction to occur.
This pathway has a smaller activation energy requirement, even though it reacts the same substances and have the same enthalpy change as the uncatalyzed reaction.
This happens because the catalyst takes part of the reaction, but it is regenerated unchanged at the end of the reaction.
Yes, economy. We know that many things we use are produced through industrial application. Thus, the slowness of a reaction would make impossible to have any kind of profit in the production of these products.
So how on earth do the guys that work in industry manage to accelerate this reactions?
They use something very important for our daily-basis called catalyst.
But what is catalyst?
Well, catalyst is a substance that increases the rate of a chemical reaction without being consumed by the reaction.
Catalyst is not only important in industry, but it also play a very important role in our body.
And how does it work?
It basically provide an alternative pathway for a reaction to occur.
This pathway has a smaller activation energy requirement, even though it reacts the same substances and have the same enthalpy change as the uncatalyzed reaction.
This happens because the catalyst takes part of the reaction, but it is regenerated unchanged at the end of the reaction.
A Reaction
Okay, now let's take all we know and try to trace what happen in a reaction:
For this let's react BrCH3(aq) and OH(aq). This will be a exothermic reaction.
First of all, for a successful reaction to occur, the particles of BrCH and OH must collapse in the correct collision geometry.
If this occur there will be enough kinetic energy to be converted into potential energy, and stored in the bonds of the particles of the activated complex.
Because the activated complex is very unstable. It can either break down in products or it can be decomposed into reactant. Remember that it is a mid term?
If the activated complex turns into product, the potential energy that was stored in the bonds of the particles, now will turn into kinetic energy as the separate. This conversion will result in the decrease of the potential energy, and rise in the temperature, which are the main characteristics of an exothermic reaction.
CHEMISTRY!
For this let's react BrCH3(aq) and OH(aq). This will be a exothermic reaction.
First of all, for a successful reaction to occur, the particles of BrCH and OH must collapse in the correct collision geometry.
If this occur there will be enough kinetic energy to be converted into potential energy, and stored in the bonds of the particles of the activated complex.
Because the activated complex is very unstable. It can either break down in products or it can be decomposed into reactant. Remember that it is a mid term?
If the activated complex turns into product, the potential energy that was stored in the bonds of the particles, now will turn into kinetic energy as the separate. This conversion will result in the decrease of the potential energy, and rise in the temperature, which are the main characteristics of an exothermic reaction.
CHEMISTRY!
Activated Complex
Remember that hill that exist in an enthalpy change diagram? Now, let's go to the top of it.
In the peak of the hill, you will find many particles that are very unstable. They do not have any completed formed bonds, nor completed broken bonds.
This particles are neither reactants, nor products, they are the mid term, a transitional specie.
This particles are referred to as the activated complex.
Activation Energy and Enthalpy
Many of you may think that activation energy can be determined by the enthalpy change of a reaction.
Well, actually there is no way you can even predict the activation energy of a reaction from its enthalpy change.
A exothermic reaction that releases a lot of energy for example, may take a long time to be completed. As well as a endothermic reaction is able to happen in the blink of a eye.
Enthalpy change is the difference in potential energy of the reactants and products and it is independent of the pathway.
The activation energy, however, is determined by the rate of temperature in a reaction.
In general, the amount of energy required to start a reaction will determine if this reaction will take long or not to occur, independent of the type of reaction it is.
Let's answer that questions:
Well, actually there is no way you can even predict the activation energy of a reaction from its enthalpy change.
A exothermic reaction that releases a lot of energy for example, may take a long time to be completed. As well as a endothermic reaction is able to happen in the blink of a eye.
Enthalpy change is the difference in potential energy of the reactants and products and it is independent of the pathway.
The activation energy, however, is determined by the rate of temperature in a reaction.
In general, the amount of energy required to start a reaction will determine if this reaction will take long or not to occur, independent of the type of reaction it is.
Let's answer that questions:
- Activation energy for a reaction is the amount of energy required for a reaction occur. Without this energy, bonds cannot be broken nor formed.
- They are two distinct concepts. However, enthalpy change is only possible to happen if the activation energy is reached.
- The spark will provide enough energy for combustion to happen.
quarta-feira, 25 de setembro de 2013
Molecular Collision
Did you know you can represent the change in the energy generated by the collisions of particles using a potential energy diagram?
 Yeah, but with one little difference:
Yeah, but with one little difference: In addition to the enthalpy change of the reaction, there will be a small hill that will represent the activation energy.
Reaction with low activation energy will occur faster than those with higher activation energy.
A explosive reaction has a very low activation energy, for example.
Temperature and Activation Energy
Hold on... doesn't temperature influence the distribution of kinetic energy?
Well, yeah, and that is why when temperature increases, the number of collisions with enough energy increases as well.
And that is also the reason for the the high rate of successful collisions at high temperature.
Well, yeah, and that is why when temperature increases, the number of collisions with enough energy increases as well.
And that is also the reason for the the high rate of successful collisions at high temperature.
Activation Energy
Let's not forget that there are two criteria to be followed, in other to a reaction occur.
We've already learned about collision geometry.
Well, now, it's time for you to learn about the Activation Energy. But... what is it?
Activation energy, or Ea, is the minimum collision energy required for a successful reaction.
When two particles collide, they will need extra energy to break down their bonds, and to form other bond for their products.
In almost every reaction, only a small number of collisions have sufficient energy for a reaction to occur.
This collision energy is based on the kinetic energy of the particles that are being collided.
As you know, temperature is a the measure of the average kinetic energy.
If you plot the number of collisions in a substance at the given temperature against the kinetic energy of each collision, you get the distribution known as Maxwell-Boltzmann distribution.
The following graphic illustrate the M-B distribution.
The dotted line indicates the activation energy.
The shaded part of the graph is represents the collisions with equal or greater energy than the activation energy.
As you can observe, they are the minority.
We've already learned about collision geometry.
Well, now, it's time for you to learn about the Activation Energy. But... what is it?
Activation energy, or Ea, is the minimum collision energy required for a successful reaction.
When two particles collide, they will need extra energy to break down their bonds, and to form other bond for their products.
In almost every reaction, only a small number of collisions have sufficient energy for a reaction to occur.
This collision energy is based on the kinetic energy of the particles that are being collided.
As you know, temperature is a the measure of the average kinetic energy.
If you plot the number of collisions in a substance at the given temperature against the kinetic energy of each collision, you get the distribution known as Maxwell-Boltzmann distribution.
The following graphic illustrate the M-B distribution.
The dotted line indicates the activation energy.
The shaded part of the graph is represents the collisions with equal or greater energy than the activation energy.
As you can observe, they are the minority.
Orientation of Reactants
Well, we've learned that one of the criteria for a effective reaction occur, is that the particles must collide with the proper orientation.
 As you can see, out of the five different types of collision, only one is geometrical, and will produce other particles.
As you can see, out of the five different types of collision, only one is geometrical, and will produce other particles.
This proper orientation is also known a collision geometry.
The following chemical equation illustrate why this criteria is essential:
NO(g) + NO3(g) = NO2(g) + NO2(g)
When the particles of NO(g) collides with particles of NO3(g), NO2(g) particles will be formed. However, not all the collisions between these particles will generate the product.
Analyse the following image
Collision Theory
Today, we will learn about Collision Theory!
Well, before we start, just stop and think about all the reactions that are happening around you in this exact moment. It is impossible! Every second, there are millions of reactions occurring around us.
When we are driving a car for example, we can analyse two different reactions: the gasoline being combusted and the steel being rusted.
Even though, these two reactions happen to be occurring in the same vehicle, one takes longer to be completed than the other.
Scientifically speaking, the combustion of gasoline has a faster reaction rate than the rusting of steel. But what is reaction rate?
Reaction rate is the change in the amount of reactants consumed or products generated over time.
However, this leaves us with a question: Why does some reactions occur faster than others?
Collision Theory
Before we answer that question above, we need to know how a reaction occurs. What causes it?
A theory explains that a reaction occur when two particles collide with each other. These particles may be atoms, molecule, or ions.
This theory is called collision theory. However, does every collision result in a reaction?
The answer for that question is NO, and here is why:
Not every collision between reactants results in a reaction.
For example, in a lab, in 1 mL sample of gas there are several collisions of particles. This number is so high that all gases' reactions would be completed in less than a second. However, gas' reactions actually take a long time to occur.
For a collision between reactants actually result in a reaction, the collision must be effective.
A effective collision, which is the one that forms products, must satisfy two criteria:
Well, before we start, just stop and think about all the reactions that are happening around you in this exact moment. It is impossible! Every second, there are millions of reactions occurring around us.
When we are driving a car for example, we can analyse two different reactions: the gasoline being combusted and the steel being rusted.
Even though, these two reactions happen to be occurring in the same vehicle, one takes longer to be completed than the other.
Scientifically speaking, the combustion of gasoline has a faster reaction rate than the rusting of steel. But what is reaction rate?
Reaction rate is the change in the amount of reactants consumed or products generated over time.
However, this leaves us with a question: Why does some reactions occur faster than others?
Collision Theory
Before we answer that question above, we need to know how a reaction occurs. What causes it?
A theory explains that a reaction occur when two particles collide with each other. These particles may be atoms, molecule, or ions.
This theory is called collision theory. However, does every collision result in a reaction?
The answer for that question is NO, and here is why:
Not every collision between reactants results in a reaction.
For example, in a lab, in 1 mL sample of gas there are several collisions of particles. This number is so high that all gases' reactions would be completed in less than a second. However, gas' reactions actually take a long time to occur.
For a collision between reactants actually result in a reaction, the collision must be effective.
A effective collision, which is the one that forms products, must satisfy two criteria:
- The correct orientation of reactants (collision geometry);
- Sufficient collision energy (activation energy, Ea).
sábado, 20 de julho de 2013
Hamlet - Character's list
- Hamlet: He is the prince of Denmark and the protagonist of the play. He is known to be thirty or sixteen years old. Hamlet is the son of the Queen Gertrude and the late King Hamlet, and nephew of the new king, Claudius. He is melancholy, bitter, cynical and full of hate to his uncle and his mother's attitude. As a reflective and thoughtful characters of a scholar boy, he is often indecisive and hesitant, but at other times prone to rash and impulsive acts.
- Claudius: He is the new King of Denmark, Hamlet's uncle, and also the play's antagonist. Hi shas lust for power and shows no signs of human feeling, however, his love for the Queen seems sincere.
- Gertrude: She is Hamlet's mother, who loves her son, however is shallow and weak. She seeks affection and status more urgent than moral. She had an affair with claudius before senior Hamlet was killed.
- Polonius: His advises the king, and he is also the father of Laertes and Ophelia.
- Horatio: He is Hamlet's best friend. He is very loyal and helpful.
- Ophelia: Hamlet's on-and-off girlfriend. She is beautiful, innocent and polite.
- Laertes: Hamlet's brotehr-in-law. They don't get along very well.
- Fortinbras: He is the prince of Norway. He is ambitious and wishes to attacks Denmark.
- The Ghost: Represents Hamlet's father. He tells the true story about his death, and his brother, Claudius.
- Rosencrantz and Guildenstern: Two friends of Hamlet from university. They are employ by the king to spy on hamlet and eventually lead him to death.
- Osric: Rich farmer, with no respect but full of power.
- Reynaldo: Poloniu's servant, who is sent to France to spy on Laertes.
Hamlet - Plot Review
During the dark nights, the ghost of the dead king walks through the ramparts of a castle of Denmark. He is first sighted by two guards, then by the scholar Horatio and, eventually, by the prince, Hamlet. Hamlet's uncle turned king after his father's death and married the queen.
When Hamlet came to know his father's ghost, they communicate and the prince becomes known that his father was murder by his uncle, Claudius. The ghost order Hamlet to seek revenge against his brother, and also murder.
Hamlet, then, devotes himself to seek for revenge against his uncle. He creates a plan which involves a fake madness. Even though the prince is looking forward to bring honor to his family's name again, he frquently delays the final act.
As Hamlet starts to act unusually, his mother and step father, which is his uncle and the king, worry about his behavior. They employ two of Hamlets friends, Rosecrantz and Guildenstern, to watch him.
Polonius, the man uncharged to advise the king and also Hamlet's father-in-law, suggests that the prince's madness was caused by love. However, Hamlet shows that he doesn't care about love at all. He even wishes that marriage was banned from the country.
Even though Hamlet heard from the Ghost the story of his ftaher's death, he is not 100% sure that story is true. He takes adavantage of a play that is coming to town and ask the actor to act a scene where the scenario is very alike the one of his father's murder. Hamlet seeks to see the king's reaction, thus, proving that he is guilty. And he was.
Claudius is consumed by guilty and fear. He decides to pray. Hamlet find him, but doesn't want to kill him, because he is praying, therefore he wouldn't go straight to heaven.
After more news that Hamlet has been acting crazy, Claudius fears his life and decides to send him to England.
When Hamlet went to confront his mother he kills Polonius, thinking that he was the king. For that he is sentenced to leave Denmark and go to England. However, the king's truly plans involve Hamlet's death.
The aftermath of Polonius death is Ophelia becoming mad and drowning herself, and the return of her brother, Laertes. He is furious, but the king is able to convince him that the responsible for his father's death is actually Hamlet. Laertes want revenge.
Laertes and the King decide to kill Hamlet, since he is coming back from England. They will start a match between him and Laertes, however, they will poisonous Laertes blade. As backup plan, the king will poising a drink and give to Hamlet.
During the match, Laertes and Hamlet are both hurt by the same poisonous blade. After Hamlet wins the match he is offered a drink, but instead his mother drinks it and dies. Hamlet kills the king. Laertes dies. Hamlet dies. The king of Norway takes the throne and treats Hamlet with respect.
domingo, 9 de junho de 2013
DNA Replication
DNA replication is the process of creating an exact copy of a molecule of DNA. This replication is said to be semi-conservative, because each new molecule of DNA contain one strand from the original molecule, and one new parent strand.
Replication starts at a specific nucleotide sequence, called replication origin. An enzyme called helicase, binds to the DNA molecule and unravel it. An enzyme called primase construct a short stand called primer. This primer will be the starting point for the attachment of new nuclotides, which is a job of the enzyme called DNA polymerase, in a process called elongation.
However, the DNA is anti parallel. The replication can occur continuously in one strand called leading strand and in other one lagging strand is replicated in short segments called Okazaki fragment. To end this process DNA ligase, spliced all this together.
Replication starts at a specific nucleotide sequence, called replication origin. An enzyme called helicase, binds to the DNA molecule and unravel it. An enzyme called primase construct a short stand called primer. This primer will be the starting point for the attachment of new nuclotides, which is a job of the enzyme called DNA polymerase, in a process called elongation.
However, the DNA is anti parallel. The replication can occur continuously in one strand called leading strand and in other one lagging strand is replicated in short segments called Okazaki fragment. To end this process DNA ligase, spliced all this together.
DNA vs RNA
- Sugar: The RNA sugar component is ribose, instead of deoxyribose.
- RNA does not have the nucleotide thymine (T), however, in its place is the nucleotide uracil (U).
- RNA is single stranded.
Structure of DNA
DNA is formed by long chains of nucleotides. Each nucleotide is formed by a phosphate group, a five-carbon sugar (deoxyribose), and a base, which can be adenine (A), guanine (G), cytosine (C), and thymine (T).
Chargaff's rule affirms that the amount of Adenine and Thymine, as well as guanine and cytosine, are always going to be similar.
The chains of nucleotides that form DNA are bound together in spiral shape. Giving it the name of double helix.
In DNA, there is a pattern called complementary base pair, which says that adenine will always bound with thymine, while guanine will always bound with cytosine.
The two strands of DNA are antiparallel, which means they run in different directions.
Meiosis
Meiosis is the process that produces haploid cells from diploid cells. Meiosis have two key outcomes:


- Reduction Division: It produces daughter cells with fewer chromosomes than the parent cells.
- Recombination: The products of meiosis have different combinations of genes. Genetic recombination give rises to genetically distinct offspring.
Meiosis is divided into two phases: Meiosis 1 and meiosis 2.
Interphase: Interphase is the same for both meiosis and mitosis. There is the growth, genetic duplication, and formation of structures necessaries for duplication.
Prophase 1: In prophase 1, each pair of homologous chromosomes, in this case non-sister chromatids, align side by side, forming the synapsis. non-sister chromatids exchange pieces of chromosomes, in a process of crossing over.
Metaphase 1: The spindle fibre guide the tetrads to the center of the cell. There they line up in homologous pairs. Each homologous of the pair is positioned in each side of the equator.
Anaphase 1: Homologous pairs are divided from one another, generating lonely chromosomes.
Telophase 1: DNA is not duplicated in this part.
Meiosis 2: Identical process as mitosis.
Nondisjunction
Sometimes, chromosomes or chromatids do not separate as they should during anaphase 1 or anaphase 2.
- In Anaphase 1, nondisjunction occurs when homologous chromosomes pairs do not separate to opposites poles, instead they all go to they same pole.
- In anaphase 2, nondisjunction occurs when sisterchromatids, fail to be divided, and they go to the same pole together.
Mitosis
The process of cell division have important functions, which are growth of the body, replacement, and repair. To accomplish each of these function, each daughter cell must have exactly the same genetic information as the parents. For this to happen, the genetic material of the parent cell must be correctly duplicated, chromatin must be condensed into chromosomes, and one set of chromosomes must be divided into each of two nuclei.
- Prophase: This is the first phase of the four of mitosis. during prophase, the chromatin condenses into chromosome. The nuclear membrane breaks down, as well as the nucleolus. Centrioles start to move to the poles, and spindles fibres start to be formed.
- Metaphase: The spindle fibres guides the chromosome to the center of the cell, the equator line. Each sister chromatid faces one pole.
- Anaphase: centromeres are splits apart and the sister chromatids separate from each other.
- Telophase: This is the final phase of mitosis. Chromatids starts to transform into chromatids. Nuclear membrane and nucleolus are back. Centrioles divids.
- Cytokinesis: The cytoplasm divides.
Cell Cycle
Cell cycle is the life cycle of a cell. Their duration vary among the different types of cells. A single cell cycle is the set of events before each division. It relates a parent cell, which is the original cell and the daughter cells, which are the new cells that a are formed in each cell division.
The cell cycle is divided into two phases: The growth phase and the division phase. Each of them are divided in other phases.
The Growth Phase

The growth phases is also called the interphase, and it is divided into 4 distinct phases.
The cell cycle is divided into two phases: The growth phase and the division phase. Each of them are divided in other phases.
The Growth Phase

The growth phases is also called the interphase, and it is divided into 4 distinct phases.
- G1 (gap 1) phase: The G1 phase is the period where the cell grows.
- G0 phase: This only happens in certain cells. G0 phase is basically a phase where the cell stop growing and doesn't look forward to divide.
- S (synthesis) phase: In this phases the DNA is copied
- G2 (gap 2) phase: Period at which there is more growth of the cell, as well as other structures are formed.
The Division Phase
The division phase is divided into two phases:
- Mitosis: The division of genetic material and the contents of the nucleus into two different sets.
- Cytokinesis: The division of the the cytoplasm and the other organelles.
Technologies
- in vitro fertilization: This process is also called, fertilization in test tube.Which means that fertilization occurs in a solution that is not inside the mother. Once cleavage happens, the embryo is introduced to the uterus for implantation.
- Ultrasound: High frequency waves are passed over the fetus to determine fetal position or size.
- Amniocentesis: Needle is inserted into the uterus, and some cells from the amniotic fluid are remove. This process is used to identify any type of abnormalities.
- CVS: Chorionic cells are analyzed.
- GIFT: Solution with sperm and ovum is mix and then transferred to the fallopian tubule.
Lactation
Lactation is controlled by hormone. Prolactin, the hormone needed for milk production, is not secreted during pregnancy, due to the high levels of estrogen and progesterone. After birth, however, the pituitary of the mother starts to produce prolactin. Thus, milk is made.
When the baby sucks the mother's nipples, sensory neurons send the information to the hypothalamus, that will produce oxytocin, and then send to the pituitary, whom will release it. The oxytocin stimulates the movement of the milk, by causing the mammary lobules to contract.
When the baby sucks the mother's nipples, sensory neurons send the information to the hypothalamus, that will produce oxytocin, and then send to the pituitary, whom will release it. The oxytocin stimulates the movement of the milk, by causing the mammary lobules to contract.
Labour
Labour is also known as parturition. This event typically begins with uterine contractions.
Uterine contraction occurs during the whole pregnancy, however, as close as it gets to the big moment, the contractions become stronger and more frequently.
The onset of labour includes hormonal and neural components. Everything start by the stretch of the cervix, which starts the release of the hormone oxytocin, from the posterior pituitary gland. The oxytocin stimulates the contraction, that will bring the baby downwards, stretching the cervix even more, repeating the cycle.
Uterine contraction occurs during the whole pregnancy, however, as close as it gets to the big moment, the contractions become stronger and more frequently.
The onset of labour includes hormonal and neural components. Everything start by the stretch of the cervix, which starts the release of the hormone oxytocin, from the posterior pituitary gland. The oxytocin stimulates the contraction, that will bring the baby downwards, stretching the cervix even more, repeating the cycle.
Teratogens
As well as the mother is able to pass nutrients and other beneficial substance, It is possible for the mother to pass harmful substances that can be very bad for the fetus.
Almost all the substances that the mother ingest or inhales can be passed through the placenta. This is specially significant in the first nine weeks, when the embryo is very sensitive to environmental factors.
Teratogens is the name given to any substance that causes structural abnormality to the baby. Cigarette smoke, for example, prevents enough oxygen to reach the baby.
One of the most damaging teratogens is called alcohol. Alcohol can affect the fetus brain, nervous system and physical development. The term that is used to describe all alcoholic disorders is called FASD. This includes the fetal alcohol syndrome (FAS).
Some prescriptions of medicines are consider teratogens.
Almost all the substances that the mother ingest or inhales can be passed through the placenta. This is specially significant in the first nine weeks, when the embryo is very sensitive to environmental factors.
Teratogens is the name given to any substance that causes structural abnormality to the baby. Cigarette smoke, for example, prevents enough oxygen to reach the baby.
One of the most damaging teratogens is called alcohol. Alcohol can affect the fetus brain, nervous system and physical development. The term that is used to describe all alcoholic disorders is called FASD. This includes the fetal alcohol syndrome (FAS).
Some prescriptions of medicines are consider teratogens.
Structures of Support
- Amnion: Transparent disk that form from cells of the embryonic disk. It surrounds the embryo completely and it is only penetrated by the umbilical cord. The amnion is filled with the amniotic fluid that protects the baby from injures and drastic temperature changes.
- Yolk sac: Yolk sac in humans is responsible to provide the embryo blood cell, while it can produce by its own. It also contributes for the formation of the digestive tract.
- Allantois: Part of the umbilical cord. It is used to clean the waste of the baby.
- Chorion: The membrane that is part of the placenta.
- Placenta: disk shaped organ that is rich in blood-vessels. It transport nutrients to the fetus, cleans its waste. transport oxygen, secretes hormone, and transport antibodies from the mother into the fetus.
Embryonic Development
In medicine, the prebirth period is divided into three trimesters. They are divided into two periods:
 In the female body, after the egg is release of the follicle, thanks to the hormone LH, it start its journey to the uterus in the oviduct, with the help of muscular contractions and the wavelike movements of the scilia, that lines in the walls of the oviduct. It takes about four days to reach the uterus, so the egg needs to be fertilized in this period of time.
In the female body, after the egg is release of the follicle, thanks to the hormone LH, it start its journey to the uterus in the oviduct, with the help of muscular contractions and the wavelike movements of the scilia, that lines in the walls of the oviduct. It takes about four days to reach the uterus, so the egg needs to be fertilized in this period of time.
 As the egg is being fertilized, it keeps moving in the oviduct. During the journey to the uterus, many events can be observed in the zygote.
As the egg is being fertilized, it keeps moving in the oviduct. During the journey to the uterus, many events can be observed in the zygote.
 The balstocyst will attach to the endometrium, with the inner cell mass against it. The trophoblast will secrete enzymes that digest some of the tissues and blood vessels of the endometrium. In a process called implatation, the blastocystslowly sinks into the endometrium. After this process is done, the woman is said to be pregnant.
The balstocyst will attach to the endometrium, with the inner cell mass against it. The trophoblast will secrete enzymes that digest some of the tissues and blood vessels of the endometrium. In a process called implatation, the blastocystslowly sinks into the endometrium. After this process is done, the woman is said to be pregnant.
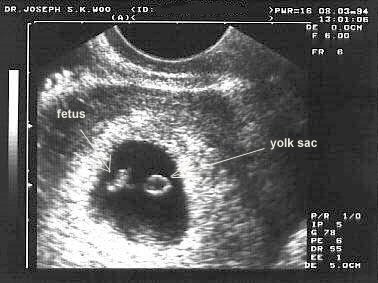
 When the process of implantation is completed, the inner mass cell starts to change. A space starts to be formed between the trophobalst and the inner mass cells. This space is called amniotic cavity, and will soon be filled with fluid, which the embryo will be floated in.
When the process of implantation is completed, the inner mass cell starts to change. A space starts to be formed between the trophobalst and the inner mass cells. This space is called amniotic cavity, and will soon be filled with fluid, which the embryo will be floated in.
 During the thrid week, a group of cells starts to be developed along the back of the embryonic disk. This cells are gonna be transformed in the baby's back and also a structure called notochord. The notochord will be the basic framework of the skeleton. The nervous system develops from the ectoderm that is just aboce the notochord. The cells above the notochord begin to thick, than they will form a tube, this tube will form the brain and the spinal cord. This process of the formation of this tube is called neurulation, and starts the organ formation. Soon after the neurulation, the heart starts to be formed.
During the thrid week, a group of cells starts to be developed along the back of the embryonic disk. This cells are gonna be transformed in the baby's back and also a structure called notochord. The notochord will be the basic framework of the skeleton. The nervous system develops from the ectoderm that is just aboce the notochord. The cells above the notochord begin to thick, than they will form a tube, this tube will form the brain and the spinal cord. This process of the formation of this tube is called neurulation, and starts the organ formation. Soon after the neurulation, the heart starts to be formed.
- Embryonic period of development: This period of development takes place in the first two third of the first trimester, which means the first 8 weeks. During this time, cells are been divided and tissues and organs are been formed, as well as structures that support and nourish the developing embryo.
- Fetal period of development: This period starts in the ninth month until the birth. During the fetal period, the body growth rapidly and organs start to work, formatting organ systems.
Fertilization
Fertilization is the starting point of the human development. It involves the joining of male and female gametes to form a single cell that have 46 chromosomes, 23 from each parent.
 In the female body, after the egg is release of the follicle, thanks to the hormone LH, it start its journey to the uterus in the oviduct, with the help of muscular contractions and the wavelike movements of the scilia, that lines in the walls of the oviduct. It takes about four days to reach the uterus, so the egg needs to be fertilized in this period of time.
In the female body, after the egg is release of the follicle, thanks to the hormone LH, it start its journey to the uterus in the oviduct, with the help of muscular contractions and the wavelike movements of the scilia, that lines in the walls of the oviduct. It takes about four days to reach the uterus, so the egg needs to be fertilized in this period of time.
Millions of sperms get into the vagina, they need to survive a long path until reach the ovum. Some die due to acid in the vagina, or because of the female's immune system, some however, take the wrong way.
The ovum is protected by a membrane that also provides it energy. In order to penetrate this membrane, the sperm cells come with the acrosome, that have enzymes. Hundreds of sperm are necessary to make a pathway through the membrane, that's why the first sperm to reach the ovum, is not always the first one to get inside it. Once one sperm cells get inside, the membrane of the ovum depolarize and prevent the other perms to get in.
As time passes by the genetic material of the gametes mix and his result in the formation of a zygote.
Cleavage
 As the egg is being fertilized, it keeps moving in the oviduct. During the journey to the uterus, many events can be observed in the zygote.
As the egg is being fertilized, it keeps moving in the oviduct. During the journey to the uterus, many events can be observed in the zygote.
In short period of time, the zygote start to divide into different numbers of cells. It divides so fast that the size of the zygote remains the same. The process of division without the enlargement of the cells is called cleavage.
When the number of cells reach 16, the zygote is now called morula. When the morula reaches the uterus it starts to be filled by liquid from uterus. As the fluids gets in the embryo, it start a formation of two different group of cells.
The entire structure is called blastocyst. One group of cells called trophoblast forms the outer layer of the blastocyst. The trophoblast will form a membrane called chorion, that will become part of the placenta.
The other group of cells are called the inner cell mass, or embryoblast. They will develop in the embryo.
 The balstocyst will attach to the endometrium, with the inner cell mass against it. The trophoblast will secrete enzymes that digest some of the tissues and blood vessels of the endometrium. In a process called implatation, the blastocystslowly sinks into the endometrium. After this process is done, the woman is said to be pregnant.
The balstocyst will attach to the endometrium, with the inner cell mass against it. The trophoblast will secrete enzymes that digest some of the tissues and blood vessels of the endometrium. In a process called implatation, the blastocystslowly sinks into the endometrium. After this process is done, the woman is said to be pregnant.
About the time that the implantation occurs, the trophoblast starts to secret the hormone hCG. This hormone has the same function as the LH. It maintains the corpus luteum alive, that will keep the level of estrogen and progesterone high, which will prevent the endometrium to be broken down. After a couple of months, the corpus luteum can be degenerated, since the placenta can secret enough estrogen and progesterone by its own.
Tissue Formation
 When the process of implantation is completed, the inner mass cell starts to change. A space starts to be formed between the trophobalst and the inner mass cells. This space is called amniotic cavity, and will soon be filled with fluid, which the embryo will be floated in.
When the process of implantation is completed, the inner mass cell starts to change. A space starts to be formed between the trophobalst and the inner mass cells. This space is called amniotic cavity, and will soon be filled with fluid, which the embryo will be floated in.
As the amniotic cavity forms, the inner mass cells start to from a disk, called the embryonic disk. The embryonic disk is formed by three layer: ectoderm, endoderm, and mesoderm. These are called the primary germ layers, and the process of their formation is called gastrulation. The disk is naw called gastrula.
The ectoderm will form our skin, nervous tissue, teeth, eye lens, and etc. The mesoderm will form the blood vessels, muscles tissues, bones, heart and etc. The endoderm will form the respiratory and digestive tract, among other structures.
Gastrulation marks the initiation of the morphogenesis, that is the series of events that form distinct structures of the developing organism. Differentiation is the cellular process which enables the cells to develops in a certain shape and perform a certain function.
Organ Formation
After the 8 week, 90% of the organs are formed, and the embryo can now be called fetus.
Assinar:
Comentários (Atom)